AG Prof. Dr. Wiebke Herzog
The group of Professor Wiebke Herzog is interested in signaling pathways regulating the growth and remodeling of blood vessels during embryonic development and regeneration, with a special focus on blood vessels of the brain and the process of endothelial cell migration.
Ongoing projects
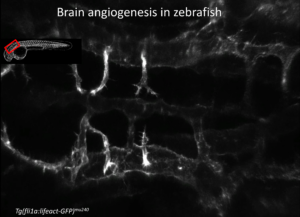
We could show that both processes rely on tight interplay of the Wnt and the S1P-signaling pathways and are currently addressing the molecular interactions by which brain angiogenesis is balanced with formation of a tight BBB.
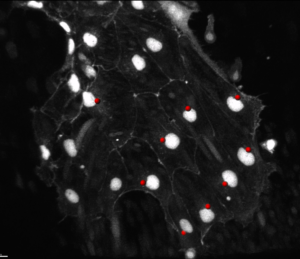
To analyze the factors regulating the migration behavior of endothelial cells in healthy and disease conditions we are using zebrafish embryos, which either have genetic defects or in which we impaired gene function or regulatory pathways pharmacologically or by morpholino injection. We are continuously identifying new potential regulators of endothelial cell migration from comparative gene expression profiling.
Live imaging of the endothelial cell behavior as well as molecular analysis of gene and protein expression (changes) will allow for new insights into whether these candidate factors regulate correct or aberrant endothelial migration and pattern formation and therefore will help to identify potential therapeutic interventions.
We currently address how Vascular endothelial cadherin (Ve-Cadherin) regulates the cell-polarity and migration directionality in collectively migrating human or zebrafish endothelial cells .
- Das R.N., Tevet Y, Safriel S, Han Y, Moshe N, Lambiase G, Bassi I, Nicenboim J, Brückner M, Hirsch D, Eilam-Altstadter R, Herzog W, Avaram R, Poss KD, & Yaniv K (2022) Generation of specialized blood vessels through transdifferentiation of lymphatic endothelial cells. Nature, 606(7914):570-575. doi: 10.1038/s41586-022-04766-2.
- Kempers L, Wakayama Y, van der Bijl I, Furumaya C, De Cuyper IM, Jongejan A, Kat M, van Stalborch AD, van Boxtel AL, Hubert M, Geerts D, van Buul JD, de Korte D, Herzog W*, Margadant C. (2021) The endosomal RIN2/Rab5C machinery prevents VEGFR2 degradation to control gene expression and tip cell identity during angiogenesis. Angiogenesis, 24(3):695-714. doi: 10.1007/s10456-021-09788-4.
* both last authors contributed equally - Quiñonez-Silvero C, Hübner K, Herzog W (2020) Development of the brain vasculature and the blood-brain barrier in zebrafish. Dev Biol. 457(2):181-190. doi: 10.1016/j.ydbio.2019.03.005
- Hübner K, Cabochette P, Diéguez-Hurtado R, Wiesner C, Wakayama Y, Grassme KS, Hubert M, Guenther S, Belting HG, Affolter M, Adams RH, Vanhollebeke B, and Herzog W (2018) Wnt/β-catenin signaling regulates VE-cadherin-mediated anastomosis of brain capillaries by counteracting S1pr1 signaling. Nat Commun. 9:4860. doi: 10.1038/s41467-018-07302-x
- Hamm MJ, Kirchmaier BC and Herzog W (2016) Sema3d controls collective endothelial cell migration by distinct mechanisms via Nrp1 and PlxnD1., J Cell Biol. 215:415-430.
- Helker C.S., Schuermann A., Pollmann C., Chng S.C., Kiefer F., Reversade B. and Herzog W. (2015) The hormonal peptide Elabela guides angioblasts to the midline during vasculogenesis. eLife, 4. doi: 10.7554/eLife.06726.